AN OPTISURG COMPANY
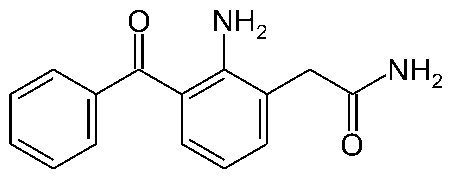

EVONAP-FORTE (Nepafenac Ophthalmic Suspension 0.3%) is a sterile, topical, nonsteroidal anti-inflammatory (NSAID) prodrug for ophthalmic use. After topical ocular dosing, Nepafenac penetrates the cornea and is converted by ocular tissue hydrolases to amfenac, a nonsteroidal anti-inflammatory drug. Nepafenac and amfenac are thought to inhibit the action of prostaglandin H synthase (cyclooxygenase), an enzyme required for prostaglandin production.
EVONAP-FORTE (Nepafenac ophthalmic suspension) is indicated for the treatment of pain and inflammation associated with cataract surgery.
One drop of EVONAP-FORTE® (Nepafenac ophthalmic suspension) should be applied to the affected eye one-time-daily beginning 1 day prior to cataract surgery, continued on the day of surgery and through the first 2 weeks of the postoperative period. An additional drop should be administered 30 to 120 minutes prior to surgery.
Use with Other Topical Ophthalmic Medication.
EVONAP-FORTE® (Nepafenac ophthalmic suspension) may be administered in conjunction with other topical ophthalmic medications such as beta-blockers, carbonic anhydrase inhibitors. alpha-agonists, cycloplegics, and mydriatics. If more than one topical ophthalmic medication is being used, the medicines must be administered at least 5 minutes apart.
EVONAP-FORTE (nepafenac ophthalmic suspension) 0.3% is supplied sterile in natural LDPE plastic bottle with dropper with grey high impact polystyrene (HIPS) cap in 3ml pack.