AN OPTISURG COMPANY
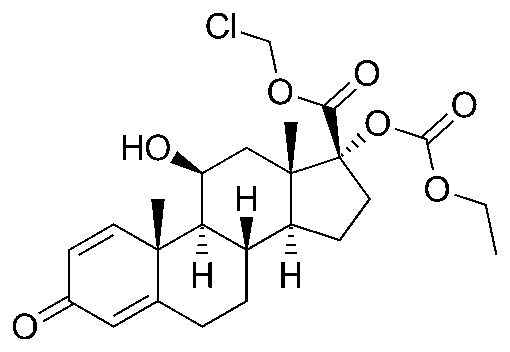
MEDILOT-T® (loteprednol etabonate and tobramycin ophthalmic suspension), is a sterile, multiple dose topical anti-inflammatory corticosteroid and antibiotic combination for ophthalmic use. Both loteprednol etabonate and tobramycin are white to off-white powders. Corticosteroids inhibit the inflammatory response to a variety of inciting agents and probably delay or slow healing. They inhibit the edema, fibrin deposition, capillary dilation, leukocyte migration, capillary proliferation, fibroblast proliferation, deposition of collagen, and scar formation associated with inflammation. There is no generally accepted explanation for the mechanism of action of ocular corticosteroids. Corticosteroids are capable of producing a rise in intraocular pressure. Loteprednol etabonate is structurally similar to other corticosteroids. However, the number 20 position ketone group is absent. It is highly lipid soluble which enhances its penetration into cells. Loteprednol etabonate is synthesized through structural modifications of prednisolone-related compounds so that it will undergo a predictable transformation to an inactive metabolite.
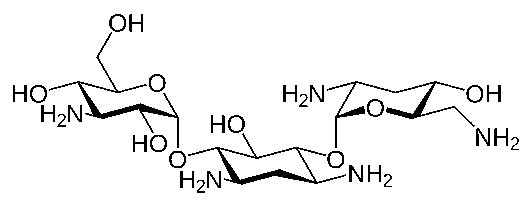
The antibiotic component in the combination (tobramycin) is included to provide action against susceptible organisms. In vitro studies have demonstrated that tobramycin is active against susceptible strains of the following microorganisms:
Staphylococci, including S. aureus and S. epidermidis (coagulase positive and coagulase negative), including penicillin-resistant strains. Streptococci, including some of the Group A-beta-hemolytic species, some nonhemolytic species, and some Streptococcus pneumoniae. Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes, Proteus mirabilis, Morganellamorganii, most Proteus vulgaris strains, Haemophilusinfluenzaand H. aegyptius, Moraxella lacunata, Acinetobacter calcoaceticus and some Neisseria species.
MEDILOT-T® is indicated for steroid-responsive inflammatory ocular conditions for which a corticosteroid is indicated and where superficial bacterial ocular infection or a risk of bacterial ocular infection exists. Ocular steroids are indicated in inflammatory conditions of the palpebral and bulbar conjunctiva, cornea and anterior segment of the globe such as allergic conjunctivitis, acne rosacea, superficial punctate keratitis, herpes zoster keratitis, iritis, cyclitis, and where the inherent risk of steroid use in certain infective conjunctivitis is accepted to obtain a diminution in edema and inflammation. They are also indicated in chronic anterior uveitis and corneal injury from chemical, radiation or thermal burns, or penetration of foreign bodies.
The use of a combination drug with an anti-infective component is indicated where the risk of superficial ocular infection is high or where there is an expectation that potentially dangerous numbers of bacteria will be present in the eye.
The particular anti-infective drug in this product (tobramycin) is active against the following common bacterial eye pathogens:
Staphylococci, including S. aureus and S. epidermidis (coagulase positive and coagulase negative), including penicillin-resistant strains. Streptococci, including some of the Group A-beta-hemolytic species, some nonhemolytic species, and some Streptococcus pneumoniae. Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes, Proteus mirabilis, Morganellamorganii, most Proteus vulgaris strains, Haemophilus influenzae, and H. aegyptius, Moraxella lacunata, Acinetobacter calcoaceticusand some Neisseria species.
Apply one or two drops of MEDILOT-T into the conjunctival sac of the affected eye(s) every four to six hours. During the initial 24 to 48 hours, the dosing may be increased, to every one to two hours. Frequency should be decreased gradually as warranted by improvement in clinical signs. Care should be taken not to discontinue therapy prematurely. Not more than 20 ml should be prescribed initially and the prescription should not be refilled without further evaluation as outlined in PRECAUTIONS above.
MEDILOT-T (combination of loteprednol 0.5% & tobramycin 0.3% ophthalmic suspension) is supplied sterile in opaque white LDPE plastic bottle with dropper with white high impact polystyrene (HIPS) cap in 5ml pack.